ISO 7886-1 Testing Sterile Hypodermic Syringes
ISO 7886-1 ANNEXES AND MEASUREMENTS
The ISO 7886-1 standard regulates the mechanical properties of hypodermic syringes and outlines the specifications for single use hypodermic syringes. It also includes information related to the design, manufacture, and functionality of the devices, while the annexes of the standard describe the standard operating procedure for testing these devices. Whereas ISO 7886-1:1993 discusses mechanical testing in annex G, the newer version of the standard, ISO 7886-1:2017 discusses it in annexes D and E.
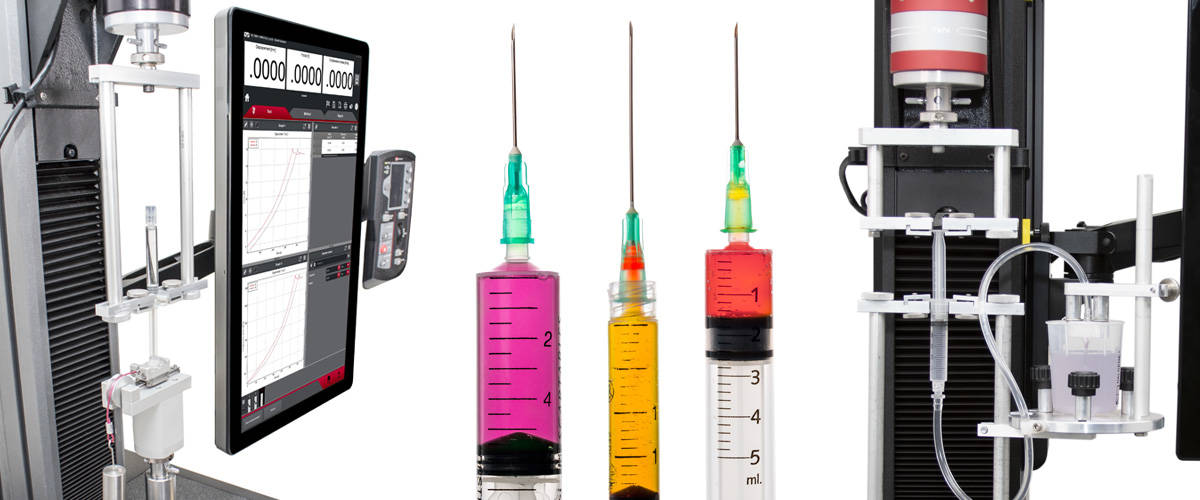
Annex D measures the quality of the plunger stopper seal by simultaneously applying a perpendicular force to the syringe barrel and maintaining a constant compressive force on the syringe piston. A visual check is performed to ensure that no liquid escapes past the plunger stopper seal. This annex is less commonly tested than Annex E, which measures the forces needed to operate the syringe plunger with water in the barrel. The force profile typically displays an initial peak force known as the break loose force, and an average force of the remaining plunger travel, known as the glide force. This test also requires the recording of maximum force during plunger travel excluding the break loose force.
Testing System
ISO 7886-1 testing is performed on a single column universal testing system such as those found in Instron's 3400 or 6800 Series. A 2870-003 syringe fixture is required and is designed for this method specifically. Because the forces involved are relatively small, a 100 N or 50 N load cell is preferred. A biotray may also be helpful since there is potential for spillage from the reservoir onto the test system frame.
最高クラスの6800シリーズ試験機のカタログ
インストロン6800シリーズ万能材料試験機は、他に類のない精度と信頼性を提供します。特許申請中のオペレーター保護機能に基づき、最新のスマートクローズエアキットおよび衝突緩和機能を搭載した6800シリーズは、材料試験をかつてないほどシンプルに、スマートに、安全にします。
Bluehill Universalのカタログ
Bluehill Universalソフトウェアは、タッチ操作と直感的なユーザーエクスペリエンスを念頭に構築されています。標準装備の試験メソッド、数秒で行われるQuickTest、強化されたデータエクスポート、そしてサービスとの直接通信を提供する新機能Instron Connectなどの機能が、これまでよりもシンプルでスマートな試験を可能にします。Bluehill 2やBluehill 3などの旧バージョンソフトウェアからは、簡単に最新バージョンのBluehillにアップグレードできます。
2870-003注射器試験治具(ISO 7886-1対応)
Instron®注射器試験治具は、滅菌の使い捨て皮下注射器に関するISO 7886-1:2017附属書E、およびISO 7886-1:1993附属書Gの試験要件事項を満たすように設計されています。