
DRUG DELIVERY DEVICE AND CONTAINER TESTING
The global demand for pharmaceutical products is on the rise, fueled by higher incidence rates of chronic disease, an aging population, and the threat of future pandemics. Patients and physicians expect drug delivery containers to be devoid of defects, capable of securing the medicine throughout its transportation and deployment. Failures can pose a severe risk to the patient or result in the disposal of large quantities of much needed supplies. For these reasons, drug delivery containers and devices require stringent evaluation, enforced by global regulatory bodies, to ensure they can perform as intended. There are multiple types of drug containers designed to store and deliver medicine under varying circumstances. This page differentiates the various containers and identifies the unique testing objectives and challenges associated with each. For more information on other biomedical applications, visit our Biomedical Industry page.
SYRINGE TESTING
Because they are responsible for delivering many different drugs in many different settings, syringes are manufactured in several varieties. Glass syringes represent the largest percentage of the market, closely followed by plastic, with a smaller market for stainless steel syringes. Despite their inherent simplicity, there are hundreds of design considerations that must be made when creating these products, as they serve as both containers and drug delivery devices. Mechanical testing aims to address many of these and assist manufacturers in achieving optimal performance and maintaining production quality.
Device usability is one of the most important design considerations. For syringes, this means the ease of dispensing fluid by using the plunger. Achieving optimal performance can require modifications to barrel geometric tolerances, inner surface roughness, siliconization processes, plunger stopper geometry, and more. Manufacturers must lean on mechanical testing during the R&D process to qualify and evaluate all design decisions.
Another important consideration is the closure and safety mechanisms for the device. Some glass needles have staked needles embedded in the cone of the barrel and require a needle shield, while most syringes use a luer connection to attach a cap and separate needle. Safety mechanisms can be classified as active or passive, depending on the interaction of the user. Active safety requires a distinct action from the operator such as placing a shield over an exposed needle. Passive mechanisms typically use a spring to hide or cover the needle after use to prevent sharps injuries. In both type of mechanism, one of the main testing challenges is alignment. Concentricity errors can affect the force and torque values of the measurement. Self-centering grips are ideal for these applications and remove opportunities for operator error.
Both prefilled glass syringes and cartridges can be directly placed into automatic drug delivery devices. These devices are designed to mechanically engage drug delivery after a patient presses a button or engages a needle shield. During activation, a loaded spring pushes a plunger down on the container and dispenses fluid. Automatic drug delivery devices dramatically increase ease-of-use for the patient, but also present additional design complexity that necessitates the evaluation of many subcomponents and functionalities. Many customers address these with multiple testing systems, each designated for a different evaluation, such as ejected volume or needle length insertion depth. Manufacturers can improve throughput, reduce operator influence, and simplify testing workflows by utilizing a single turnkey solution to perform all the required functional tests.

 ISO 7886-1 Testing Sterile Hypodermic Syringes
ISO 7886-1 Testing Sterile Hypodermic Syringes
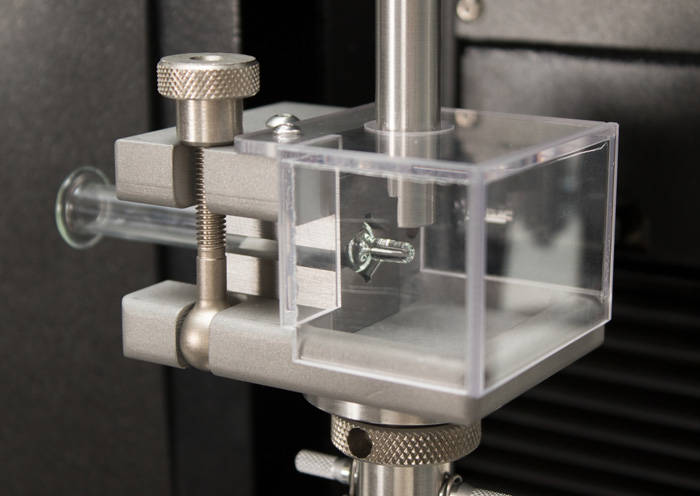 ISO 11040 - Design and Functional Properties of Prefilled Syringes
ISO 11040 - Design and Functional Properties of Prefilled Syringes
 ISO 80369 - Small Bore Connectors for Liquids
ISO 80369 - Small Bore Connectors for Liquids
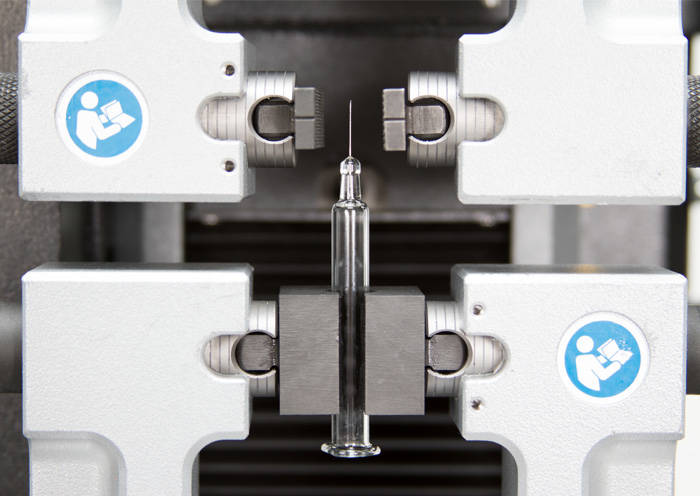 Syringe Needle Testing
Syringe Needle Testing
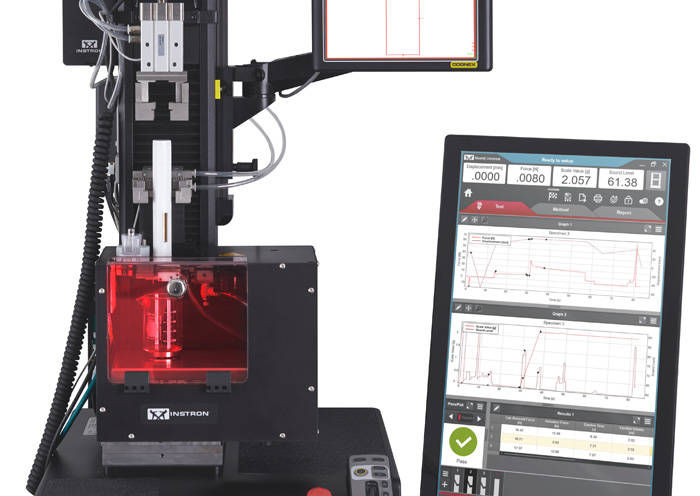 ISO 11608-1:2014 Needle-Based Injection Systemsa
ISO 11608-1:2014 Needle-Based Injection Systemsa
CARTRIDGE TESTING
Cartridges are most commonly used within automated drug delivery systems including autoinjectors, pen injectors, and wearables. They present unique advantages for pharmaceutical companies, being simpler to manufacture than syringes and more compact for improved storage efficiency. They are also simple in design, consisting of a borosilicate glass container with elastomeric seal and stopper, enclosed by an aluminum cap. The cartridge relies on two external mechanisms to deliver medicine – a manual or automated action to actuate the plunger, and an attachable needle to channel the medicine into the body. Most testing on cartridges evaluates the former, ensuring the forces necessary to dispense fluid can be achieved within the device.

Like syringes, alignment is a primary challenge for achieving repeatable testing results. Alignment concerns can be mitigated by using self-aligning fixtures and the ability to make XY adjustments to the workspace. The geometry of cartridges presents a key difference in the fixturing required for testing. They do not have finger flanges, meaning all gripping force must be applied to the sides of the container. The clamping forces must be calibrated to prevent damage to the container, which is made of glass. The use of an insert to support the bottom of the cartridge can limit the application of side loads and result in better repeatability in testing.
VIAL TESTING
Vials are storage containers for pharmaceuticals and are not part of the actual drug delivery. They generally consist of a glass container, rubber seal, and an aluminum crimped cap. A syringe is used to puncture the rubber seal and draw out the precise dosage required for administration. Of concern to vial manufacturers is the container closure integrity (CCI), a series of assessments to validate the closure prevents outside particulates from contaminating the product. These tests themselves are not mechanical in nature and use electrical or pressurized means to determine the closure integrity. Instron systems help provide empirical evidence identifying the key mechanical parameters for the elastomeric seal and crimping procedure that correlate to CCI performance. Residual Seal Force (RSF) is one of the main tests that is performed, determining the force required to overcome the internal forces of the elastomeric seal, which maintains contact between the vial and cap. This test is vital for evaluating the many variables which can ultimately effect CCI:

- Time from Sealing
- Storage Temperature
- Storage Humidity
- Stopper Design
- Stopper Material
- Crimping Technique
The main challenges related to RSF testing are ensuring proper alignment of the device and the evaluation of the RSF point on the load displacement curve. While many operators will manually select the point on the curve, Bluehill software allows the operator to add first and second derivative measurements, allowing for the automatic recognition and selection of the inflection points associated with RSF.
Additional testing is performed related to physician use and labelling. Instron's custom products group has designed specialized fixtures to repeatably remove the plastic cap and peel off the label, as it is important to ensure the adhesive strength can be maintained through hot, cold, or fluctuating storage temperatures.
IMPROVING EFFICIENCY AND REPEATABILITY
As you move towards commercialization of a product, the quantity of testing will inevitably increase. Efficiency and repeatability are two critical parameters that can be affected by increased volume. To address this, there are many solutions which can be utilized to improve process flow and reduce operator errors. These solutions can include:
- Cobots
- Automated XY Stages
- Barcoding
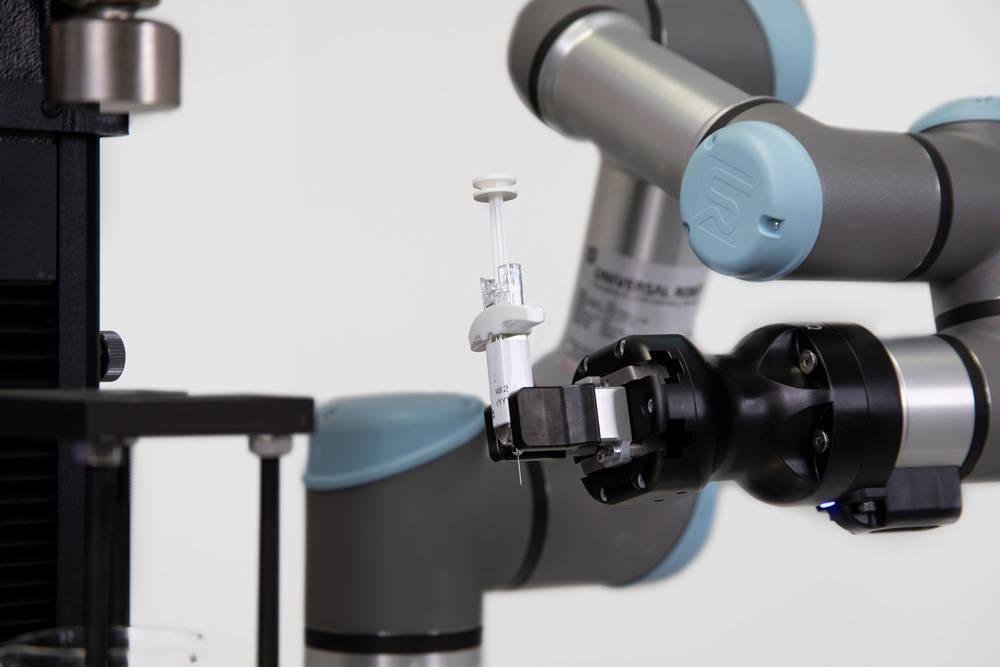
Real World Scenario: Design verification testing for example, is always the final push before receiving product approval, but may only occur a few times a year. Ensuring the lab has the appropriate staffing to both complete all the necessary testing and produce the documentation and analysis can be a challenge. Utilizing automation can help meet these challenges, removing the need for operators to stand in front of the system, and instead concurrently work on other required tasks. Automation like the CT-6 cobot can help optimize the efficiency of the lab, allowing a lab manager to appropriately distribute the workload and meet the tight deadlines associate with product release.