
TESTING MEDICAL CONSUMABLES
Medical consumables represent the largest category of biomedical testing and include a wide variety of single-use products such as surgical tools, PPE, wound closure products, specimen collection products, and more. Healthcare settings rely on single-use products to mitigate the occurrence of hospital acquired infections and ensure patient safety. These products are typically either FDA class I or II medical devices, which, despite having less stringent test requirements than higher risk products, are often produced in such large quantities that specific steps must be taken to support high volume mechanical testing. To compensate for the larger volumes, throughput and repeatability become critical test requirements addressed through specialized fixturing, efficient operator workflows, and intuitive software. Another critical component within this category is the medical grade packaging used for finished goods, which has its own set of testing requirements. For more information on other biomedical applications, visit our Biomedical Industry page.

 ASTM F88 - Testing Seal Strength of Flexible Barrier Materials
ASTM F88 - Testing Seal Strength of Flexible Barrier Materials 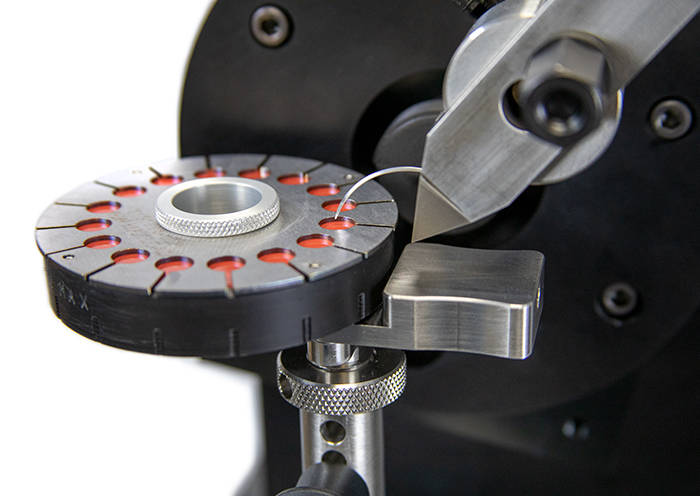 ASTM F3014 - Curved Needle Testing
ASTM F3014 - Curved Needle Testing  PPE Testing
PPE Testing  EN455-2, ISO 11193, ASTM D6319 - Tensile Testing of Medical Gloves
EN455-2, ISO 11193, ASTM D6319 - Tensile Testing of Medical Gloves  Tensile Testing Surgical Sutures
Tensile Testing Surgical Sutures  ASTM D7860 - Torque Retention for Child Resistant Packaging
ASTM D7860 - Torque Retention for Child Resistant Packaging  ISO 10555 - Tensile Testing of Catheter Tubing
ISO 10555 - Tensile Testing of Catheter Tubing PRODUCT TESTING SPECTRUM
Evaluation of these devices can occur at many points throughout the product development and production processes, meaning the actual test sample can range in complexity from raw materials to subcomponents and finished goods. At the raw material stage, testing methodology is often much more prescriptive to well-defined standards and known fixturing solutions. In many cases, standard tensile grips or compression platens can be utilized to properly evaluate different materials and help engineers make informed decision regarding material selection for their product. At the component level of testing, unique product geometry creates a greater need for customized fixturing. Utilizing XY tables or tapped test plates provide users the flexibility to adapt their system to many different types of components. These fixtures also enable you to ensure that the loading axis is properly aligned with the feature of interest on the product. Components may need to be evaluated in many different ways order to fully characterize the performance of the device. A full performance assessment may also require a qualitative assessment of the failure mode in addition to the measured load and displacement data. Integrated testcams can be helpful in providing visual feedback of the failure event synchronized with the test data.
 ISO 7886-1 Testing Sterile Hypodermic Syringes
ISO 7886-1 Testing Sterile Hypodermic Syringes
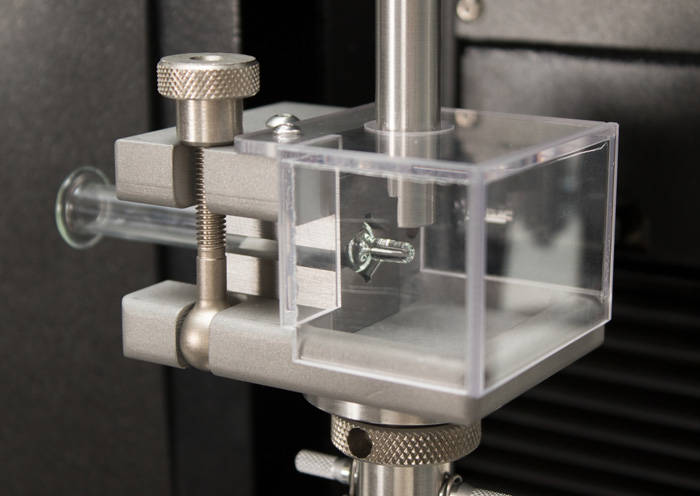 ISO 11040 - Design and Functional Properties of Prefilled Syringes
ISO 11040 - Design and Functional Properties of Prefilled Syringes
 ISO 80369 - Small Bore Connectors for Liquids
ISO 80369 - Small Bore Connectors for Liquids
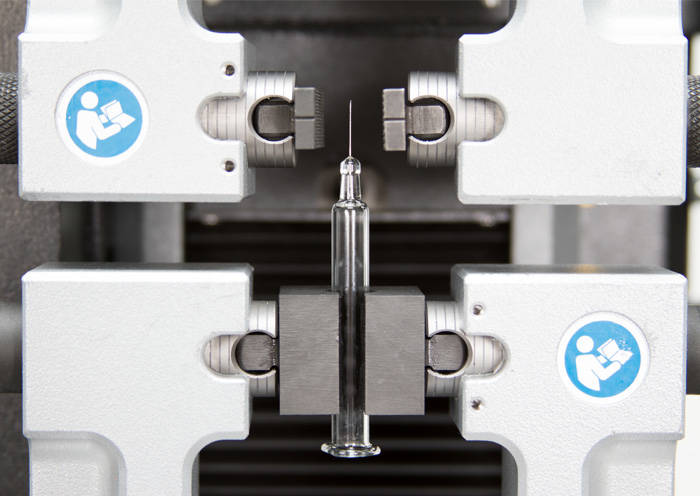 Syringe Needle Testing
Syringe Needle Testing
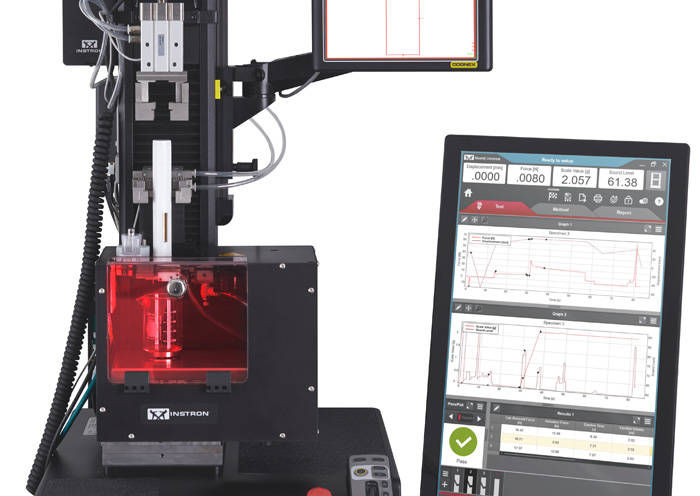 ISO 11608-1:2014 Needle-Based Injection Systemsa
ISO 11608-1:2014 Needle-Based Injection Systemsa
- Cobots
- Automated XY Stages
- Barcoding
- Automated Specimen Measurement Devices
- Bluehill® Workflows

PACKAGING
Medical products require rrobust airtight packaging to ensure that device sterilization is maintained until the time of use. This packaging is critical to prevent any contamination, which would ultimately result in a patient acquiring an infection. To verify the strength of the packaging seal, most customers test to ASTM F88, which provides the testing criteria for evaluating the seal. The main challenges are specimen preparation and data collection. Many specimen preparation fixtures exist to uniformly produce the 1 inch wide strips necessary per the standard. Seal strength testing will result in a non-uniform curve with instantaneous changes requiring a high enough data capture rate to ensure you are accurately recording the event. If the data capture rate is too low, the load curve could be rounded off and result in an artifically low average seal strength. Pneumatic grips with flat faces are ideal for repeatably holding on to the specimen tails without damaging the material in any way.
SUSTAINABILITY FOCUS
Hospitals are increasingly concerned with the waste issues brought about by their consumption of single-use medical products. The development of bioplastics is a burgeoning industry with a strong market need, and standards are currently in development to address a range of biopolymer properties across many healthcare applications. In the meantime, many typical plastics standards such as ASTM D638 and ISO 527 have updated their verbiage to include bioplastics and 3D printed plastics. The testing requirements for these standards are well known, and by using them for evaluation researchers can clearly distinguish between the material properties of petroleum and those of biologically-based plastics. Researcher may need to perform tensile, compression, flexure, torsion, and other forms of testing on these new sustainable materials in order to properly characterize them. R&D facilities need the tools at their disposal to perform these comprehensive analyses and clear the regulatory hurdles that the FDA and other international organizations will create.
