Personal Protective Equipment (PPE) Testing
Materials Testing Guidance for Personal Protective Equipment
The global demand for medical equipment has skyrocketed in response to the COVID-19 pandemic, with many companies refocusing their efforts to produce personal protective equipment (PPE). With this vast uptick in demand, medical device manufacturers have scrambled to increase their production capacities while non-medical manufacturing companies have transitioned their own production facilities to create items such as masks, gloves, and nasal swabs. With increased manufacturing comes increased quality control testing, and Instron has received numerous inquiries from companies seeking to expand their testing capacity or reconfigure their existing equipment to test PPE. This guide was created to help familiarize manufacturers with key testing requirements and provide an overview of current FDA regulations. We hope that it will be a useful resource for anyone seeking to aid the fight against COVID-19.
Medical Masks
Medical masks come in two primary types: single-use surgical masks and N95 respirator masks. Surgical masks are intended to prevent viral spread by containing droplets produced by the wearer, while respirator masks are designed to protect the wearer from virus particles that have been aerosolized. Both of these masks are relatively easy to manufacture and test, and many textile manufacturers have shifted their operations to produce them in an attempt to meet the current demand. Many of these companies already own materials testing equipment and are able to make small modifications to their existing systems in order to perform the required FDA testing.
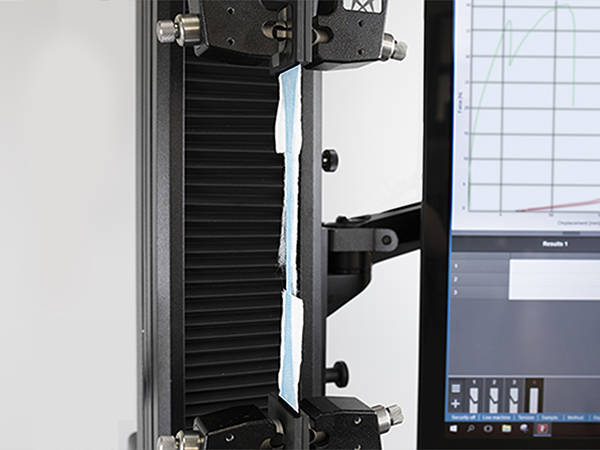
Fabric Test
Mask fabric is generally tested in accordance with general textile standards such as ASTM D5034. Because fabric samples are prone to jaw breaks, we recommend pneumatic grips with smooth jaw faces to minimize this risk. In order to capture peaks and troughs generated by individual fiber breaks, we recommend a test system with a high data capture rate, such as Instron's 68SC-5.

Elastic Test
It is important to test the strength of the connection between a mask's fabric and the elastic band that holds it into place. This test is performed by loading the band to a minimum of 10 N and visually evaluating it to ensure there has been no separation. With masks now being worn for longer periods of time than ever, it may also be valuable to perform a relaxation test to determine its durability.

Filter Test
Respirator masks must be tested to ensure the strength of connection between the mask fabric and the respirator valve. This test can be accomplished using a side-acting grip on the base of the system and a custom-made hook fixture attached to the load cell.
Medical Gloves
ASTM classifies medical gloves according to their material (latex, nitrile, natural rubber, PVC, or polychloroprene), while ISO classifies them based on their application (patient examination or surgical). Regardless of the testing standard, material, or clinical application, the equipment and general procedure for testing is consistent across all medical glove types. ASTM D6319, ISO 11193, and EN 455-2 are standards used by the biomedical industry to regulate the tensile properties of medical gloves. The key results for all glove testing standards are the tensile strength and ultimate elongation of the material. Rather than testing the entire glove, a dogbone specimen is cut from the finished glove and testing in accordance to the relevant elastomeric standard (ASTM D412 or ISO 37).
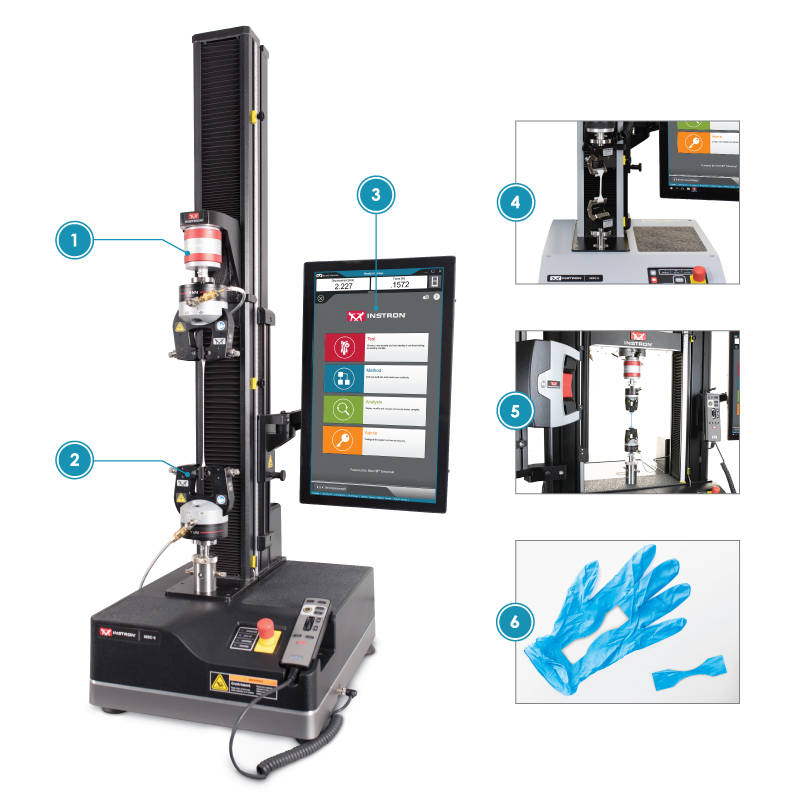
| Glove Test Setup | |
|---|---|
| 1) | Load Cell A 500N load cell is an appropriate capacity for all glove materials. |
| 2) | Pneumatic Grips Air pressurized grips ensure consistent clamping forces Jaw faces are easily interchangeable to ensure the correct surface texture is used for the material. Elastomeric materials like rubber gloves typically require rubber coated faces due to how thin the specimen is. The rubber coating is able to prevent slippage of the material without damaging the specimen. |
| 3) | Bluehill Software The biomedical method suite includes preconfigured methods for EN455-2 |
| 4) | Elastomeric Roller Grips Roller grips provide a cost effective gripping solution for thin elastomers The roller grip utilizes a proportional clamping pressure which increases as more force is applied to the specimen |
| 5) | AVE 2.0 An optical non-contacting strain device can be used to ensure more accurate strain measurement |
| 6) | Specimen Preparation All the major ASTM/ISO/EN standards require a dumbbell shaped specimen to be stamped from the palm of the glove EN 455-2 takes into consideration the potential discrepancies in thickness between the palm and the fingertips. The standard compares their thickness and uses a correction factor for the tensile strength of the speicmen. |
Nasal Swabs
Nasopharyngeal (NP) swabs are crucial tools in the diagnosis of influenza and respiratory diseases. Despite their similar appearance, these swabs are considerably more specialized than the standard cotton swabs used for personal hygiene, using synthetic fibers for the swab staff and tiny bristles for the swab tip. In an effort to bolster the global supply, significant collaborations have occurred between 3D printer manufacturers and medical research teams, which have resulted in a massive increase in production capacity of test quality NP swabs. It is critical to perform mechanical testing to determine if the performance of the 3D printed swabs is comparable to the performance of those produced by standard methods.

3 Point Bend Test
Nasal Swabs are subjected to flexural forces as they travel through the nasal passage. 3 point bend tests help characterize these stresses. It is also important to evaluate the weak point at the tip of the swab which helps achieve the correct size for transport. Instron's standard 2810-400 3 point bend fixture with 10 mm diameter anvils is ideal for these applications.
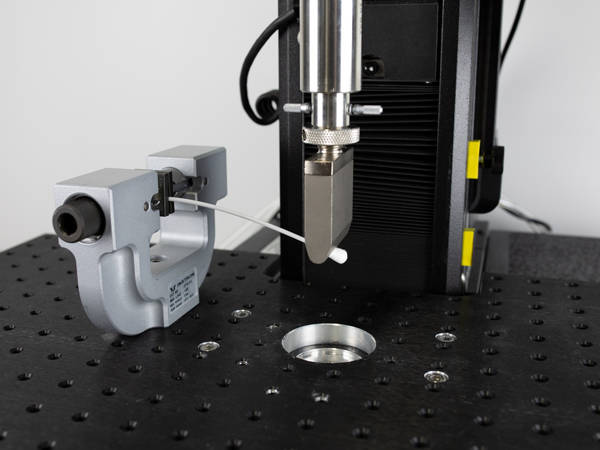
Cantilever Bend
The cantilever bend test best represents the stresses seen when the swab is held during the procedure. The material needs to be flexible enough to ensure it will not fail during the test. This setup is accomplished using a component test plate and an advanced screw action grip to hold the specimen in place. Any probe can be used to deflect the tip of the swab.

Tip Shear Strength
The tip of an NP swab is made of tiny bristles that allow for maximum sample collection. These bristles need to withstand shear forces as they move across the walls of the nasal passage. In order to test this property, the tip and base of the swab are clamped with advanced screw action grips to evaluate the maximum force required to break the bristles or the tip itself.
The Food and Drug Administration is the main regulatory body overseeing the production and distribution of PPE in the United States. The level of FDA involvement depends on the class of the device, which can range from class 1 to class 3 based on the device’s potential risk of nonconformance. Most types of PPE are labeled as class 1 devices, which have the fewest barriers to approval.
Because it can take months or even years to gain FDA approval, in times of health crisis the FDA issues something called an Emergency Use Authorization (EUA). An EUA essentially loosens the requirements for production and distribution of certain medical products to allow production to ramp up quickly. EUAs are currently being granted to manufacturers of COVID test kits, virus therapies, ventilators, respirators, and PPE. These emergency authorizations are generally granted to specific companies who apply to expedite the approval process, but they are also being released as blanket statements covering certain types of PPE so that smaller companies can also participate with minimal red tape. The EUAs include additional documentation that outline the enforcement policy for PPE manufacturing during the current public health emergency and provides criteria for quality control standards as well as the required labeling of products released under the authorization.



