
How the Bluehill® Traceability Module Helps Reduce Risk in Your Safety Critical Industry
To understand the value of Bluehill’s Traceability module to the wider materials testing industry, it is helpful to first understand why the FDA (Food and Drug Administration) requires medical device and pharmaceutical companies operating in the U.S. to comply with 21 CFR Part 11, which is just one of many regulations Traceability is designed to address.
Introduced in 1996, Part 11 acts as a guidance for companies to implement electronic records and digital signatures in place of paper-based equivalents. While the move from paper to digital records can be an intimidating process change for a laboratory, the long-term cost and security benefits have motivated much of the biomedical industry to make the switch. However, conforming to the digital age is not the only, or even the primary reason that Part 11 compliance provides value to a lab. The fundamental value realized by this high level of regulation is a superior level of confidence in the product being manufactured.
The cost of failure for a “bad batch” of medical equipment can be enormous – not only for the company, but also for the end consumer whose life is negatively impacted by the failed product. People rely on products such as stents, sutures, and syringes to perform as expected, and the FDA’s job is to protect the public’s well-being by providing guidance and rules that must be followed by any business selling products into the industry.
Whenever a medical product fails during use, an investigation is carried out to determine cause and liability. If a company’s product is deemed liable for damages, the company will likely pay a hefty toll through a combination of recalls, litigation, and damage to their brand image. As the industry’s cost of failure is high, biomedical companies are financially motivated to comply with regulations and invest in process improvements to ensure product quality.
Costly failures are not exclusive to the biomedical industry, however. They occur whenever the use of a product or service results in an undesirable experience by its user. Sometimes this cost is modest; for example, if a kitchen garbage bag prematurely tears while under load. In this case the customer will likely take extra care when filling their trash, and if premature tearing continues to occur despite precautions they might submit a complaint, demand a refund, or even refuse to purchase the brand again. If this is an isolated occurrence, the manufacturer’s profits will barely see a dent. However, if the root cause of the failure is more systemic, many customers may be affected and the manufacturer might need to take a more costly corrective action. Even so, the relative cost of failure here is quite low, and legal action is avoided by a simple refund or replacement. Not every product failure, however, is so harmless.
When The Cost of Failure Is Too High To Do NothingMore serious product failures can result in injury or death to the user. For example, parachute manufacturers operate in a safety critical industry, and a skydiver’s life depends on each component functioning as intended. Due to a heightened focus on product safety, the rates of fatal accidents have plummeted in recent years, and according to the National Safety Council a person is more likely to die from a lightning strike than from skydiving. But however unlikely, if a component fails due to manufacturer error – say the paracord snaps under stress (see Instron origins here) – and a fatality occurs, costly litigation will ensue and the manufacturer’s brand image will likely be damaged beyond repair. In these cases the cost of failure is clearly very high to all parties involved, and companies have gone out of business after failures of this magnitude.
The severity of a product’s cost of failure is dependent on the nature of the product, how it is intended to be used, and any potential risks created by its intended use. With this in mind we have to wonder - which non-biomedical manufacturing industries must also manage a high cost of failure? Two industries immediately come to mind as being in the same high-risk category: automotive and aerospace.
Other Safety Critical Industries
The automotive industry sells cars to consumers who expect a high level of safety. When airbags are not deployed or seatbelts malfunction during a serious accident, the manufacturer will undergo scrutiny. Similarly, the aerospace industry flies billions of people around each year at 30,000 feet, all of whom expect to land safely. Aerospace companies’ financial livelihoods depend on an exceptionally high rate of uneventful flights.
Suppliers who produce materials and components for either of these industries must address the high cost of failure with appropriate business strategies. The leading strategy to mitigate the risk of failure is to invest in people, equipment, material, and processes that improve their product’s quality. For many high cost of failure industries, quality is not optional – regulatory bodies mandate it. Like the biomedical industry, the automotive and aerospace industries are both highly regulated to ensure that a reliable product reaches the end consumer.
The Role of AuditsTo ensure that regulations are being met, manufacturers undergo routine audits conducted not only by external government agencies but by their own quality organization as well. Audits prove that the current manufacturing process will result in a product of sufficient quality. Furthermore, audits provide assurance that in the case where a “bad batch” does reach the consumer, the manufacturer will be able to quickly identify the root cause so that they can understand the scope of the problem and issue corrective actions. An audit of processes involving material testing equipment will include the control of product testing methods and results. This is where Bluehill’s Traceability module enters the conversation.
Integrated with Bluehill’s standard security modes, the Traceability module allows a lab to track and review the who, what, when, and why for any action performed on their test system. To provide context to each of these questions:
- “Who?” is the user performing the action
- “What?” is the action, which includes all or any of the following:
- The creation, modification, or review of all Bluehill files (methods, samples, and report templates) or other system level actions, such as:
- Ex. 1 – a PDF test report is generated for review
- Ex. 2 – a specimen is deleted from a sample
- Ex. 3 – a test method’s crosshead rate is modified
- Ex. 4 – a test method’s yield stress calculation is modified
- Ex. 5 – a user’s permissions are modified
- See all details here
- The creation, modification, or review of all Bluehill files (methods, samples, and report templates) or other system level actions, such as:
- “When?” is the date and timestamp of the action in local time with time zone information
- “Why?” is the reason for the action, entered by the user as a comment
Note: all examples assume the user has security permission to perform the action
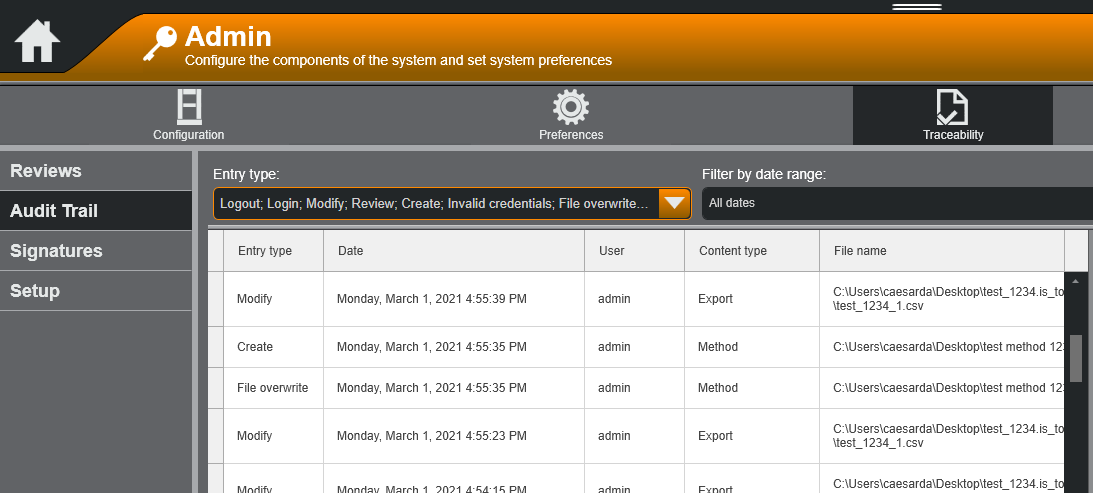
Why These Questions Matter
While not all system actions create downstream quality issues, each of the examples listed above have the potential to compromise the integrity of Instron data and devalue the results used to characterize the properties of the material or component under test. For instance, let’s play out the fourth example, where a user modifies a test method’s yield stress calculation. This change can cause the yield stress result to be artificially high for all subsequent tests. Consequently, a flawed batch can pass product tolerance checks, and it won’t be until a failure is reported in the field that a flag will be raised. Unaware of the root cause and scope of the problem, the company begins an investigation, tracing the batch back to its production and quality assurance tests performed prior to shipment. Eventually, the test method used for the bad batch will be painstakingly checked by someone attuned to the test method, and the incorrect calculation will be identified. The time elapsed between when the calculation was changed and the flag being raised might be a week, a month, or even a year. How many bad batches pass inspection during this period? The scope and cost of failure caused by a simple mistake can grow quickly.
The Insights Gained Through TraceabilityIf this scenario is replayed with the Traceability module configured on the Instron test system, the outcome will be different. With Traceability, the lab can require all revisions to methods to be electronically approved by one (or two) additional users prior to an operator being able to test specimens. If the reviewer can reject the incorrect yield stress modification to begin with, the crisis can be averted altogether. If the reviewer mistakenly approves the change, the company will not know until the same flag is raised in the field. However, in this situation, Traceability can assist the company’s investigation of the root cause. Instead of manually searching for errors in the test method from memory, the lab can efficiently identify any changes to the method by either searching the automated audit trail or viewing the revision history of the method file, both accessible within Bluehill Universal.
This example illustrates just one use case where the Traceability module can not only improve a lab’s data integrity and confidence in their product’s quality, but it can also lower the risk of a costly product failure. When we pause to look at the world around us and think about all the materials and devices that we routinely trust with our health and safety, we begin to see risk around nearly every corner. Every morning we wake up trusting our cars to transport us safely and our airplanes to stay in the sky. We trust these products to be safe in the same way that we trust our medical supplies to be safe, even though the FDA does not regulate the automotive and aerospace industries. But Part 11 compliance is not the reason why companies should be concerned about data integrity, and this is why Bluehill’s Traceability module is so valuable in any industry where high risk of failure products are manufactured. Bluehill’s Traceability module is designed to help minimize the chances of costly accidents occurring, and in doing so protect both the end user and the manufacturer from the consequences of catastrophic failure. For additional details regarding the Traceability module’s features, please navigate to the links in this article. If questions arise, please contact Instron sales for assistance.
