ISO 7864
ASTM F3014
ISO 11040
最高クラスの6800シリーズ試験機のカタログ
インストロン6800シリーズ万能材料試験機は、他に類のない精度と信頼性を提供します。特許申請中のオペレーター保護機能に基づき、最新のスマートクローズエアキットおよび衝突緩和機能を搭載した6800シリーズは、材料試験をかつてないほどシンプルに、スマートに、安全にします。
Bluehill Universalのカタログ
Bluehill Universalソフトウェアは、タッチ操作と直感的なユーザーエクスペリエンスを念頭に構築されています。標準装備の試験メソッド、数秒で行われるQuickTest、強化されたデータエクスポート、そしてサービスとの直接通信を提供する新機能Instron Connectなどの機能が、これまでよりもシンプルでスマートな試験を可能にします。Bluehill 2やBluehill 3などの旧バージョンソフトウェアからは、簡単に最新バージョンのBluehillにアップグレードできます。
Torsion Add-On 3.0
Torsion Add-On 3.0は、軸方向およびねじり試験の両方を必要とする医療機器、電子機器、消費者製品、包装、自動車部品などの性能を測定するための簡素なソリューションを提供します。
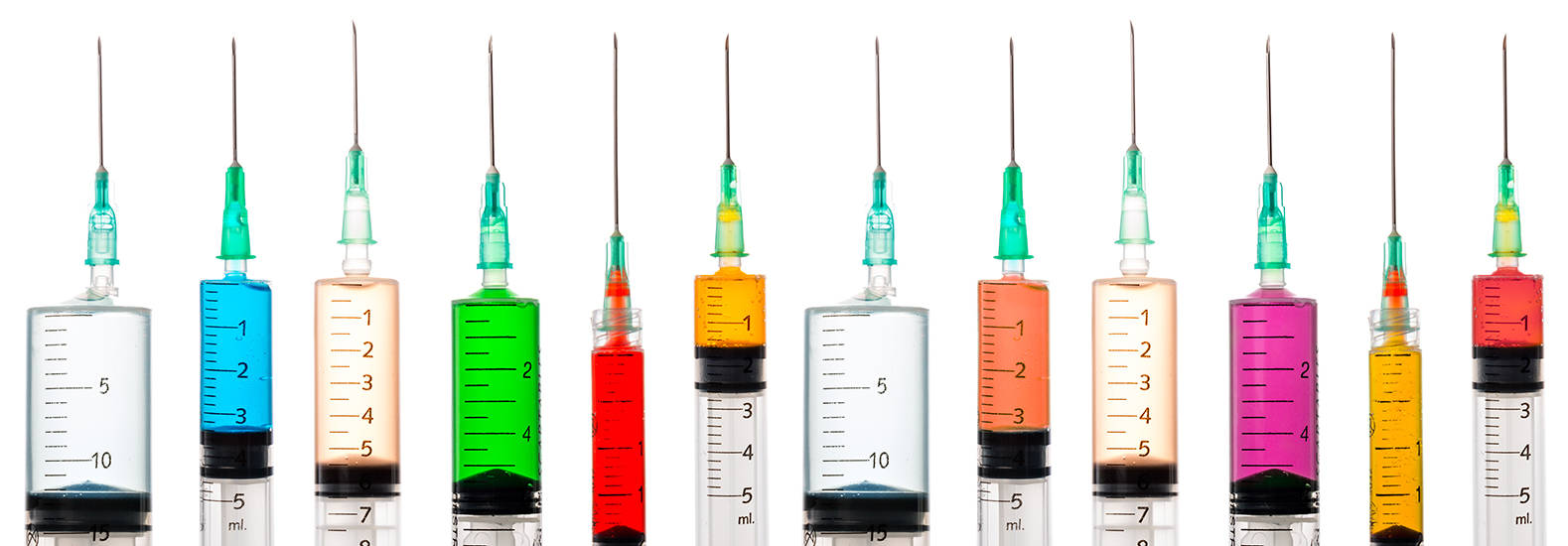
ISO 11040:薬剤充填済みガラス製注射器用の試験治具
インストロンは、ISO 11040の10種の附属書すべてに沿って試験を実施できるように設計された、モジュール式の治具を提供しています。