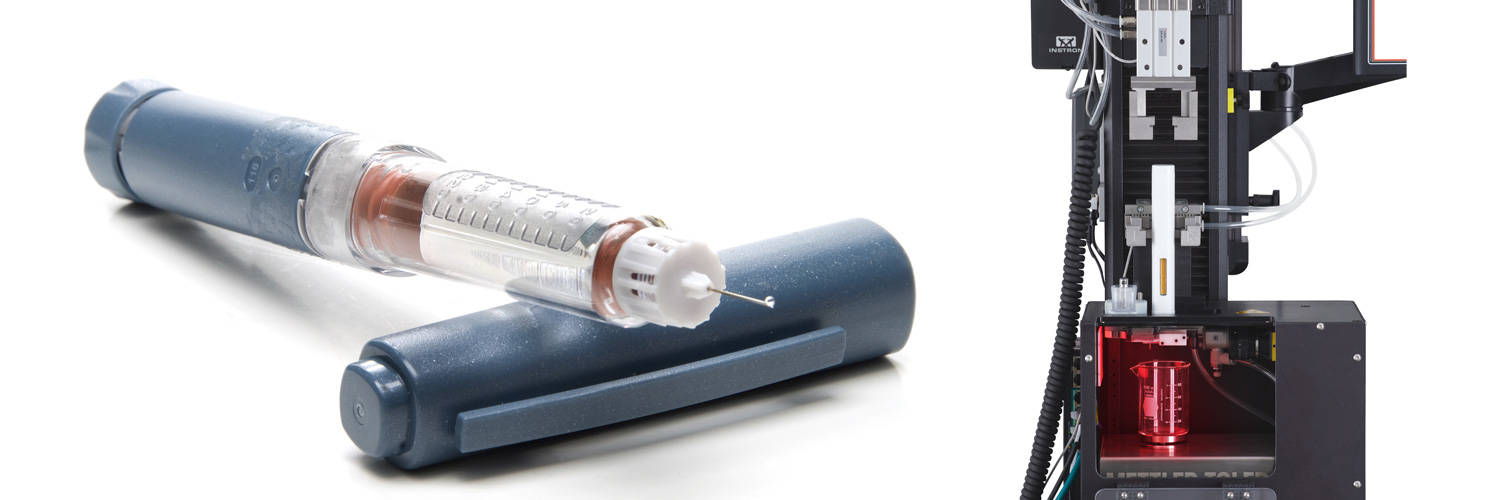
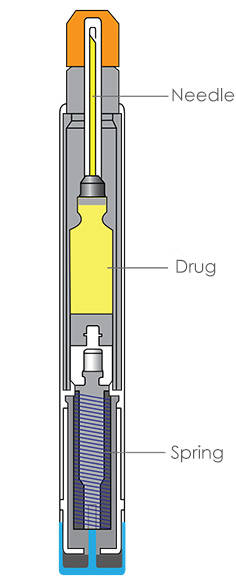
Types of Needle-Based Injection Systems
The 'needle injection systems' definition includes single and multi-dose syringe or cartridge-based systems. These devices can also be generally referred to as combination products, combining a syringe or cartridge with a mechanical device to administer the medicine. Furthermore, the devices can also be single-use or reusable. Consumers are most likely familiar with the terms "autoinjector" and "pen injector," where autoinjectors are typically a single-use single-dose device most commonly associated with epinephrine treatment of anaphylaxis, and pen injectors are typically a multi-use reusable device associated with treatment of chronic diseases like diabetes.
Mechanical Testing System
Instron has developed a single column 6800 Series testing system with an autoinjector test fixture to provide repeatable and reliable results. Utilizing a single system to sequentially test all the parameters outlined in ISO 11608-5 provides more consistent test results and simplifies data collection and analysis. Laser measurement of injection time provides the most reliable results, regardless of fluid viscosity.
最高クラスの6800シリーズ試験機のカタログ
インストロン6800シリーズ万能材料試験機は、他に類のない精度と信頼性を提供します。特許申請中のオペレーター保護機能に基づき、最新のスマートクローズエアキットおよび衝突緩和機能を搭載した6800シリーズは、材料試験をかつてないほどシンプルに、スマートに、安全にします。
Bluehill Universalのカタログ
Bluehill Universalソフトウェアは、タッチ操作と直感的なユーザーエクスペリエンスを念頭に構築されています。標準装備の試験メソッド、数秒で行われるQuickTest、強化されたデータエクスポート、そしてサービスとの直接通信を提供する新機能Instron Connectなどの機能が、これまでよりもシンプルでスマートな試験を可能にします。Bluehill 2やBluehill 3などの旧バージョンソフトウェアからは、簡単に最新バージョンのBluehillにアップグレードできます。
半自動注射器(ISO 11608-5対応)
インストロンの半自動注射器試験治具は、6800/5900試験機と、TestProfiler機能を搭載したBluehill® Universalソフトウェアとともに使用するように設計されています。これにより、キャップの取り外し、起動荷重、注射時間、注入量/ボリューム、注射針保護部の解除荷重を、1つの試験メソッド内で測定することが可能になります。